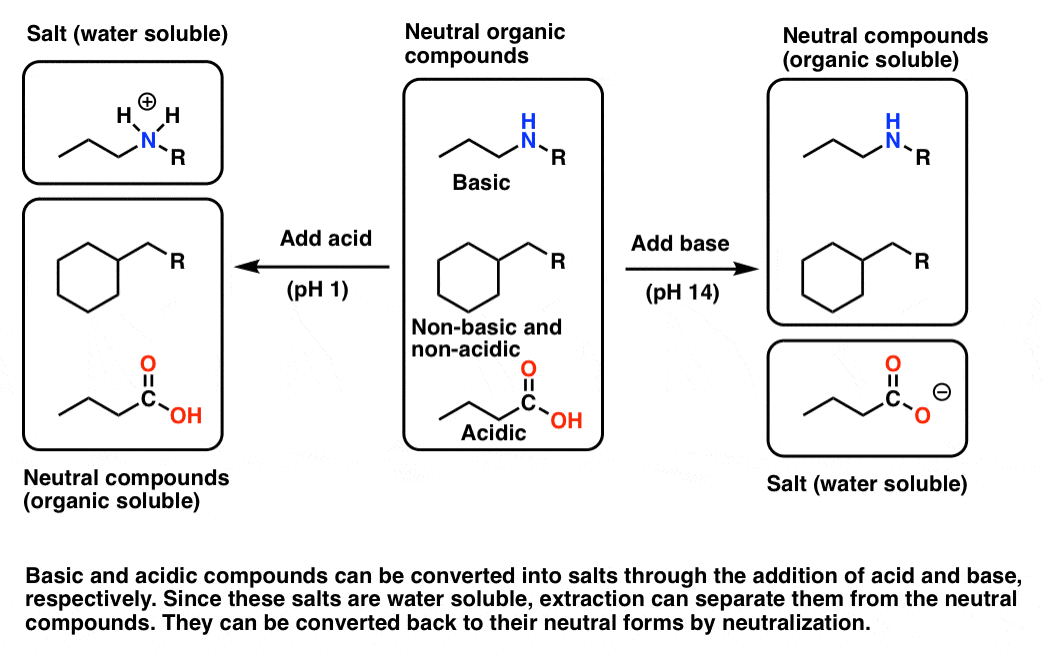
Isolation Example Chemistry . there are several reasons to use extraction in the chemistry lab. Identify which separation method is most suited for a given. the first modern chemical isolation of an element is attributed to the alchemist hennig brand (c. Describe different methods of separation. It is a principal method for isolating compounds from plant materials. however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. 1) chemical properties, 2) distillation, 3). the fourth compound is not chemically altered, but it is dissolved in an organic solvent. the point has been to give an overview of the key techniques used for purifying crude mixtures: By the end of this section, you will be able to: isolation and purification of compounds from natural products is the most important step for molecule structure. We now want to recover each compound in its.
from www.masterorganicchemistry.com
Describe different methods of separation. We now want to recover each compound in its. the first modern chemical isolation of an element is attributed to the alchemist hennig brand (c. the point has been to give an overview of the key techniques used for purifying crude mixtures: isolation and purification of compounds from natural products is the most important step for molecule structure. It is a principal method for isolating compounds from plant materials. By the end of this section, you will be able to: however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. 1) chemical properties, 2) distillation, 3). there are several reasons to use extraction in the chemistry lab.
Natural Product Isolation (2) Purification of Crude Mixtures Overview
Isolation Example Chemistry It is a principal method for isolating compounds from plant materials. We now want to recover each compound in its. the first modern chemical isolation of an element is attributed to the alchemist hennig brand (c. 1) chemical properties, 2) distillation, 3). the point has been to give an overview of the key techniques used for purifying crude mixtures: however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. Identify which separation method is most suited for a given. the fourth compound is not chemically altered, but it is dissolved in an organic solvent. By the end of this section, you will be able to: there are several reasons to use extraction in the chemistry lab. Describe different methods of separation. It is a principal method for isolating compounds from plant materials. isolation and purification of compounds from natural products is the most important step for molecule structure.
From studiousguy.com
3 Isolated System Examples in Real Life StudiousGuy Isolation Example Chemistry the fourth compound is not chemically altered, but it is dissolved in an organic solvent. isolation and purification of compounds from natural products is the most important step for molecule structure. however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. 1). Isolation Example Chemistry.
From topperscbse.com
Class 12 Chemistry Notes Chapter 5 General Principles and Processes of Isolation Example Chemistry isolation and purification of compounds from natural products is the most important step for molecule structure. Identify which separation method is most suited for a given. the fourth compound is not chemically altered, but it is dissolved in an organic solvent. We now want to recover each compound in its. the first modern chemical isolation of an. Isolation Example Chemistry.
From byjus.com
NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Isolation Example Chemistry the point has been to give an overview of the key techniques used for purifying crude mixtures: Identify which separation method is most suited for a given. the fourth compound is not chemically altered, but it is dissolved in an organic solvent. the first modern chemical isolation of an element is attributed to the alchemist hennig brand. Isolation Example Chemistry.
From ndankmari.blogspot.com
Isolation Process In Chemistry / Shallow trench isolation Revolvy Isolation Example Chemistry 1) chemical properties, 2) distillation, 3). By the end of this section, you will be able to: We now want to recover each compound in its. isolation and purification of compounds from natural products is the most important step for molecule structure. however, more often than not a procedure calls for a solution to be extracted multiple times. Isolation Example Chemistry.
From www.slideserve.com
PPT Lab 10 Colony isolation PowerPoint Presentation ID229064 Isolation Example Chemistry Describe different methods of separation. the first modern chemical isolation of an element is attributed to the alchemist hennig brand (c. We now want to recover each compound in its. isolation and purification of compounds from natural products is the most important step for molecule structure. the fourth compound is not chemically altered, but it is dissolved. Isolation Example Chemistry.
From www.masterorganicchemistry.com
Natural Product Isolation (2) Purification of Crude Mixtures Overview Isolation Example Chemistry We now want to recover each compound in its. Describe different methods of separation. however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. Identify which separation method is most suited for a given. there are several reasons to use extraction in the. Isolation Example Chemistry.
From www.studiestoday.com
CBSE Class 12 Chemistry General Principles Process of Isolation of Isolation Example Chemistry however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. the fourth compound is not chemically altered, but it is dissolved in an organic solvent. Identify which separation method is most suited for a given. We now want to recover each compound in. Isolation Example Chemistry.
From www.youtube.com
Thermodynamic tricks, open, closed & isolated system, first law YouTube Isolation Example Chemistry the fourth compound is not chemically altered, but it is dissolved in an organic solvent. however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. It is a principal method for isolating compounds from plant materials. Describe different methods of separation. By the. Isolation Example Chemistry.
From www.scribd.com
Isolation of DNA Precipitation (Chemistry) Chemistry Isolation Example Chemistry 1) chemical properties, 2) distillation, 3). Describe different methods of separation. We now want to recover each compound in its. Identify which separation method is most suited for a given. It is a principal method for isolating compounds from plant materials. the first modern chemical isolation of an element is attributed to the alchemist hennig brand (c. By the. Isolation Example Chemistry.
From ndankmari.blogspot.com
Isolation Process In Chemistry / Shallow trench isolation Revolvy Isolation Example Chemistry It is a principal method for isolating compounds from plant materials. Describe different methods of separation. isolation and purification of compounds from natural products is the most important step for molecule structure. there are several reasons to use extraction in the chemistry lab. We now want to recover each compound in its. the fourth compound is not. Isolation Example Chemistry.
From betrained.in
General Principles And Processes Of Isolation Of Elements NCERT Book Isolation Example Chemistry It is a principal method for isolating compounds from plant materials. the point has been to give an overview of the key techniques used for purifying crude mixtures: however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. 1) chemical properties, 2) distillation,. Isolation Example Chemistry.
From www.slideserve.com
PPT PHYSICALCHEMICAL ANALYSIS & ISOLATION METHODS FOR VARIOUS PHYTO Isolation Example Chemistry the fourth compound is not chemically altered, but it is dissolved in an organic solvent. Identify which separation method is most suited for a given. the first modern chemical isolation of an element is attributed to the alchemist hennig brand (c. Describe different methods of separation. isolation and purification of compounds from natural products is the most. Isolation Example Chemistry.
From betrained.in
General Principles And Processes Of Isolation Of Elements NCERT Book Isolation Example Chemistry the fourth compound is not chemically altered, but it is dissolved in an organic solvent. It is a principal method for isolating compounds from plant materials. isolation and purification of compounds from natural products is the most important step for molecule structure. the first modern chemical isolation of an element is attributed to the alchemist hennig brand. Isolation Example Chemistry.
From www.researchgate.net
Flow chart for the isolation of the compounds 13. Download Isolation Example Chemistry the fourth compound is not chemically altered, but it is dissolved in an organic solvent. the point has been to give an overview of the key techniques used for purifying crude mixtures: 1) chemical properties, 2) distillation, 3). Describe different methods of separation. It is a principal method for isolating compounds from plant materials. By the end of. Isolation Example Chemistry.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation ID2654677 Isolation Example Chemistry Describe different methods of separation. however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. there are several reasons to use extraction in the chemistry lab. Identify which separation method is most suited for a given. 1) chemical properties, 2) distillation, 3). It. Isolation Example Chemistry.
From www.masterorganicchemistry.com
Natural Product Isolation (2) Purification Techniques, An Overview Isolation Example Chemistry however, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this. isolation and purification of compounds from natural products is the most important step for molecule structure. there are several reasons to use extraction in the chemistry lab. the fourth compound is. Isolation Example Chemistry.
From www.youtube.com
Isolation of elements 01/12th chemistry unit6 YouTube Isolation Example Chemistry 1) chemical properties, 2) distillation, 3). the first modern chemical isolation of an element is attributed to the alchemist hennig brand (c. We now want to recover each compound in its. the fourth compound is not chemically altered, but it is dissolved in an organic solvent. By the end of this section, you will be able to: . Isolation Example Chemistry.
From solutionpharmacy.in
Resins Classification, Chemical Test, Extraction, and More Isolation Example Chemistry the fourth compound is not chemically altered, but it is dissolved in an organic solvent. It is a principal method for isolating compounds from plant materials. isolation and purification of compounds from natural products is the most important step for molecule structure. Identify which separation method is most suited for a given. the first modern chemical isolation. Isolation Example Chemistry.